Microbial Interactions – an alternative taxonomy
Is it mostly Darwin or mostly Marx: ‘survival of the fittest’ vs ‘socialist cooperation’
Precisely what is happening in the ‘microbial community’/microbiome is a topic of intense interest to all microbial ecologists. Multiple classes of interactions are theoretically plausible, but in my opinion we don’t know quantitatively how important each class is in structuring the community. Many people have their prejudices – mine is that microbes are ruthless predatory capitalists seeking to overwhelm their competitors, but I also admit I have little empirical evidence on authentic communities to support this. I want to eventually delve into why such evidence is difficult to obtain and then think about which systems might be least refractory to analysis. But that will be for part 2 of this topic – after some reflection, I realized I should address the taxonomy of ecological interactions and its application to microbial systems.
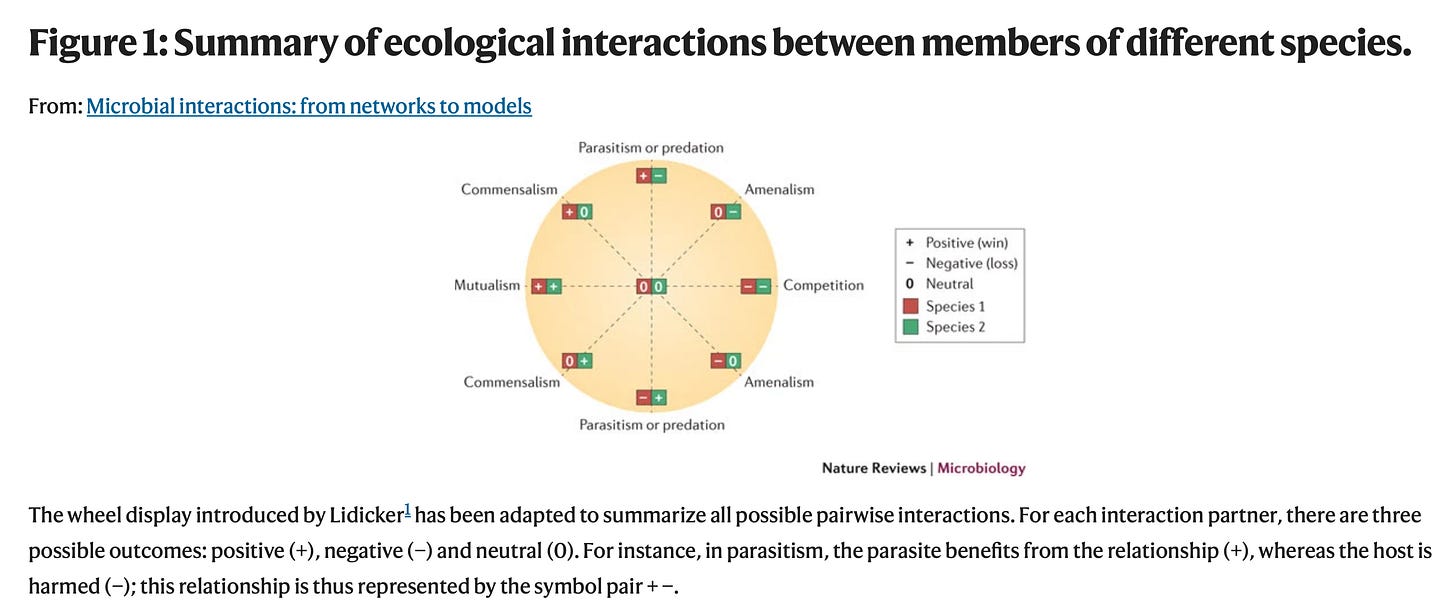
From my earliest days as a microbial ecologist, I confronted this categorization and found it mind-numbing (From Faust and Raes (2012)):
By binning every possible combination of + and – interactions (and all do exist), I didn’t feel that one gets much of a flavor as to which interactions are major players in community ecology and which are interesting sidebars. Nor does this taxonomy aid understanding of how these mechanism operate in practice.
Readers may find this equally mind-numbing, but here is my list of biotic forces that structure community composition …
A. Interspecies competition for resources
Molecules/ions: Transporters and catalysts on cell surfaces
Production of extracellular enzymes (foraging / cheaters) — Burns et al., 2013
Nutrient acquisition (siderophores)
B. Metabolic interactions
Unidirectional provision of growth factors – amino acids and vitamins
Syntrophy (thermodynamics): Interspecies H2 transfer, anaerobic methane oxidation
Cross-feeding
Redox cycles (particularly in gradient ecosystems): Fe+3/Fe+2 , SO4-2/S-2
Many mutualisms fit under this rubric – e.g., Rhizobium/legumes
Sequential utilization (metabolic feeding):
Nitrifying bacteria
Xenobiotic degradation: Anaerobic reductive dechlorination of PCB’s followed by their aerobic mineralization
Modification of local microenvironment
Reduction in pH by lactic acid bacteria
At an extreme, leads to succession (in which the modifying organism becomes extinct)
C. Trophic level interactions
‘Microbial loop’ in ecosystems (predation by protists, rotifers and nematodes as well as predatory bacteria)
Parasitism (Viruses)
D. Allelopathy (chemical warfare) / Interference competition
Bacteriocins, tailocins, type VI secretion systems and contact-dependent inhibition (Booth et al., 2023)
Plant defense compounds: phytoalexins, phenolics, terpenoids, alkaloids, flavonoids, and tannins
E. Chemical communication / signaling
Interspecies quorum sensing
Plant-microbe: Root nodules
F. Structural
Coaggregation in biofilms
Microbial mats
Lichens / mycorrhiza
Any topics missing?
Interspecies competition for nutrient resources is likely to be widespread and the most important ‘interaction’ among microbiome members, particularly to the extent that new phylotypes immigrate into the system or physicochemical conditions ‘pulse’ (Odum et al., 1995) to create variations in selective forces. Competitive outcomes when a nutrient concentration becomes growth-rate limiting entails cell membrane-bound or extracytoplasmic enzyme activities, such as the affinity of transport proteins for limiting substrates (Button, 1984), or the production of cell-surface associated or extracellular enzymes for macromolecule hydrolysis (Burns et al., 2013). Interspecific competition could also involve the production of extracellular nutrient acquisition molecules such as siderophores (Andrews et al., 2003). Particularly intriguing is the competition for soluble Fe between human transferrin and pathogens such as Pseudomonas aeruginosa, Yersinia pestis, Staphylococcus aureus and Eschericia coli.
However the catalog of microbial interactions goes far beyond simple resource competition and includes a variety of metabolic interactions. A straightforward case is where an ecotype is incapable of biosynthesis of an essential cell component (amino acid, nucleoside or vitamin). Many cultivated isolates from diverse habitats cannot grow on media with an energy source + mineral salts and bioinformatic analysis of genomes revealed ~20% were missing the genes for biosynthesis of one or more amino acids (Ramoneda et al., 2023). It remains an open question as to the source mechanism whereby these requirements are met – about 10% of aquatic primary production measured by short-term assays has been found dissolved in the aquatic milieu (although the molecular makeup of this fraction is unknown). Is ‘excretion’ by phototrophs (and chemoheterotrophs) by active (intentional) excretion, just a ‘normal’ inefficiency in retaining cytoplasmic molecules or release from cells whose cytoplasmic membrane has been damaged? Using sophisticated analystic methods, in situ B vitamin concentrations have been measured at pM levels in seawater (Sañudo-Wilhelmy et al., 2012) — but remember that the concentration depends not only upon excretion by some cells but also upon the affinity of other cells’ transporters for the molecule.
Other interactions are based on thermodynamic exigencies, as occur in syntrophic associations in which the fermentation of organic acids or alcohols is endergonic unless the metabolic end product H2 is maintained at a low partial pressure by H2-consuming microbes. Metabolic cross-feeding can occur in a number of ecosystems -- in gradient ecosystems such as thermally stratified lakes or microbial mats, redox cycling between oxidized and reduced forms (for example, sulfide and sulfate, or ferrous and ferric iron) can drive the interaction of either a chemoautotroph or photoautotroph that oxidizes the reduced molecule with a chemoheterotroph that uses the oxidized form as a terminal electron acceptor (Jorgensen et al., 2019). There are also cases of sequential utilization of substrates. A classic case is Nitrosomonas – Nitrobacter interaction in the oxidation of ammonia to nitrate. In addition, xenobiotic catabolism can occasionally involve multiple organisms (Bull and Slater, 1982).
In addition to metabolic interactions, secondary metabolites produced by one organism can serve as toxicants or chemical cues for microbes. Bacteriocins are one class of microbial defense systems. These small proteins are bactericidal for a narrow range of bacteria closely related to the producing strain. They have been found in all major lineages of Bacteria and some Archaea, and detailed analysis of their evolution and biochemistry have been carried out in a few organisms. Their ecological importance can be inferred from the broad abundance and diversity detected within cultivated microbes. However, the specific roles these molecules have in structuring microbial communities is unclear (Riley, 2002). Plants also can produce a broad array of secondary metabolites which have inhibitory effects not only against animal or other plant species but also members of the microbial community.
Not only do some organisms produce inhibitory chemicals, there are intriguing cases where chemicals serve as signals to an interacting partner. This is true for quorum sensing (Banerji et al. 2020) and also in plant interactions with microbes, in which they exude chemical cues that attract and stimulate specific microbial groups, as in the case of the legume-rhizobium symbiosis.
Physically-structured habitats such as biofilms and mats provide massive opportunities for interactions, including ones based on physical contact or construction of extracellular matrices. In my second post on microbial interactions, I want to dive more deeply into how biofilms could provide a great system to better quantitatively understand how interactions play a role in microbial community ecology – the dental plaque is an excellent example of this (Kuramitsu et al., 2007).
In ending, I would be remiss to not point out that properties unique to microbes yield yet another vast potential for interaction – horizontal gene transfer plus relatively rapid growth rate can produce evolutionary dynamics that far outpace any found in animal or plant communities. Microbes provide an avenue for experimental analysis of evolutionary ecology in a way extraordinary difficult for other organisms. That will be a topic for a future post.
References
Abisado, RG et al. (2018) Bacterial Quorum Sensing and Microbial Community Interactions. MBIO 9: e02331-17. DOI 10.1128/mBio.02331-17
Andrews SC et al. (2003) Bacterial iron homeostasis. FEMS Microbiol Rev 27: 215-237
Banerji, R et al. (2020) Role of interspecies bacterial communication in the virulence of pathogenic bacteria. Crit Rev Microbiol 46: 136-146
Booth SC et al. (2023) The evolution of short- and long-range weapons for bacterial competition Nature Ecology & Evolution 7: 2080–2091.
Burns RG et al. (2013) Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol Biochem 58: 216-234
Button DK (1984) The physical base of marine bacterial ecology. Microb Ecol 28: 273-85. doi: 10.1007/BF00166817.
Faust, K., Raes, J. (2012) Microbial interactions: from networks to models. Nat Rev Microbiol 10: 538–550. https://doi.org/10.1038/nrmicro2832
Jorgensen, BB et al. (2019) The Biogeochemical Sulfur Cycle of Marine Sediments. Front Microbiol 10. DOI10.3389/fmicb.2019.00849
Kuramitsu HK et al. (2007) Interspecies interactions within oral microbial communities. Microbiol Mol Biol Rev 71: 653-670. doi: 10.1128/MMBR.00024-07.
Odum WE et al. (1995) Nature’s pulsing paradigm. Estuaries 18: 547-555
Ramoneda J et al. (2023) Taxonomic and environmental distribution of bacterial amino acid auxotrophies. Nature Communications 14: 7608
Riley, MA and Wertz, JE (2002). Bacteriocins: Evolution, ecology, and application.
Ann Rev Microbiol. 56: 117-137
Sañudo-Wilhelmy SA, et al. (2012) Multiple B-vitamin depletion in large areas of the coastal ocean. Proc Natl Acad Sci USA. 2012;109:14041–14045. doi: 10.1073/pnas.1208755109.
Slater JH and Bull AT. (1982) Environmental microbiology: biodegradation. Phil Trans Roy Soc B: 297: 575-597. https://doi.org/10.1098/rstb.1982.0063
Your moment of Zen
I’ve been spending some time learning new ways of processing black and white images — here is a current product that is also colorized, taken at the Romanesque Abbazia Sant’Antimo near Montalcino in the Val d’Orcia, south of Siena, Italy