Introducing Think Like a Microbe
The musings of a recovering research scientist
This substack is intended as a macroscopic view of a science that is microscopic: microbial ecology. I was a practicing microbial ecologist for about 40 years and my research covered a broad swath of habitats – freshwater lakes, soils, subsurface sediments and mixed-culture bioreactors. As a ‘recovering’ research scientist, my thoughts have turned more to whether microbial ecology has a philosophy (and if it doesn’t what it should be).
What is Microbial Ecology?
“Microbial ecology” is invoked for a lot of research studies and I want to be very clear about its boundaries for the purposes of these discussions. Formally, microbial ecology is the study of microorganisms’ interactions with their environment. Those environments may be aquatic, particulate (soils or sediments) or other organisms (microbes, plants or animals). It is a fundamental science whose objective is to elucidate the principles and mechanisms that give rise to the phenomena we observe in ecosystems.
By this definition, there are other very important areas of microbiological research that are distinct from microbial ecology. One is environmental microbiology, which encompasses applied studies that determine how microbes can be used to benefit human society. Studies in this realm include biodegradation and bioremediation of xenobiotics, biogeochemical cycling, pathogen transmission in the environment, and wastewater treatment.
I also claim (perhaps controversially) that the investigation of microbial diversity in and of itself is not a component of microbial ecology. It certainly does contribute to environmental microbiology, in that it illuminates the raw material (genes and organisms) that can be exploited for new natural products or microbial activities. It does contribute greatly to our understanding of the evolution of organisms and their genes. But in the absence of connection to the resolution of an ecological question (for example, the mechanisms that generate organismal diversity in a habitat), a census of a natural microbial population does not advance microbial ecology.
The title of this Substack is purposeful in two different ways. To “think like a microbe” means perceiving the world as they do, at a spatial scale measured in micrometers not meters; at which there may be temporal and spatial heterogeneities in relevant physical and/or chemical processes. Let’s call this the microenvironment and will be an important touchstone for me in these blog posts. The second rationale to “think like a microbe” is in developing theory -- imagine optimal solutions to the problems microbes must solve to grow or to persist in their microenvironment. Of course, microbes are not sentient but evolution through natural selection of fitter types can produce solutions that strike us as well-adapted. Of course, we also find that regulatory schemes or morphological adaptations are more complicated than what we imagine are necessary to solve problems. That may be a clue that the adaptive landscape those microbes are facing is different or more complex than we have thought.
Microbial ecosystems
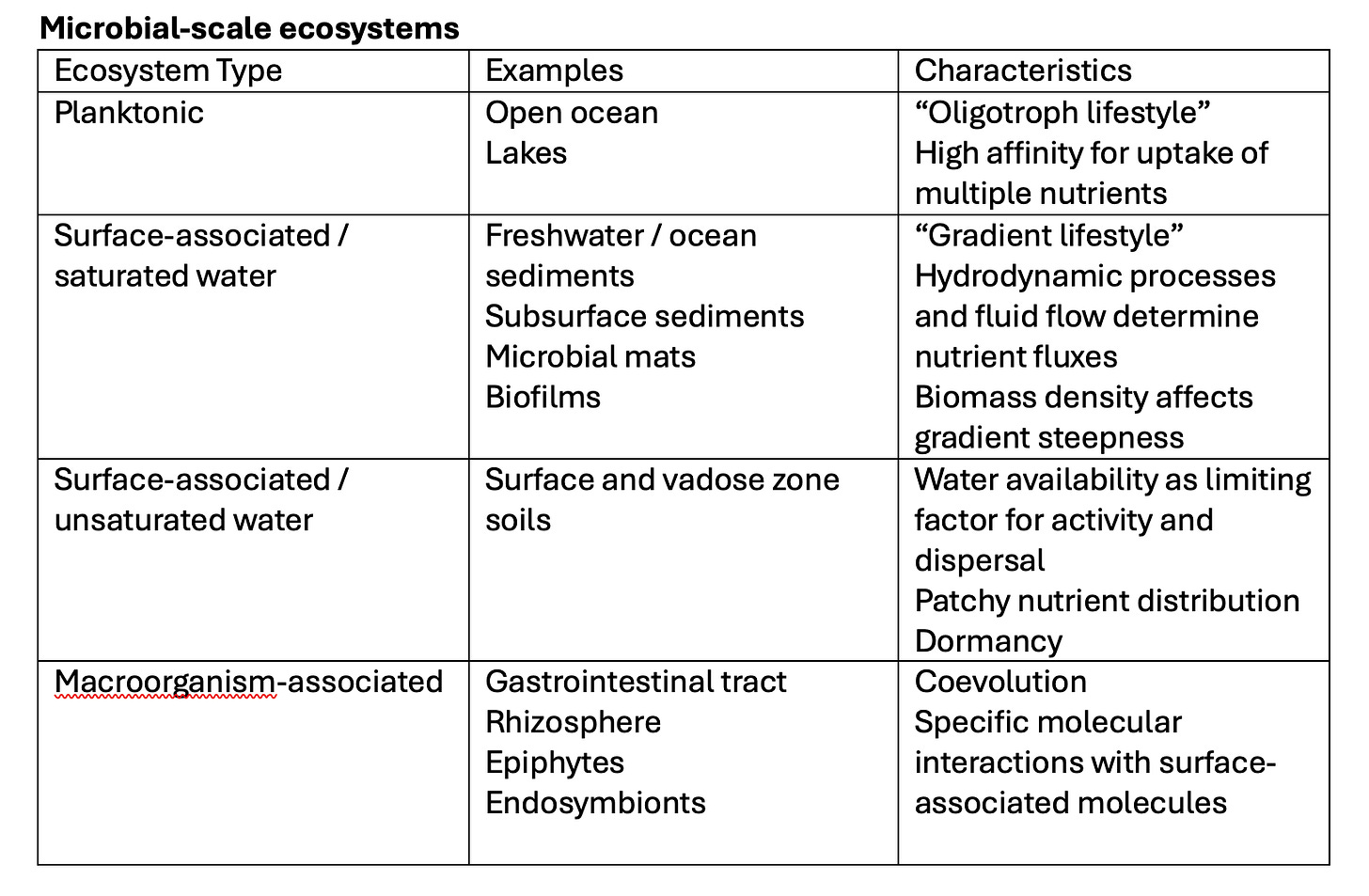
These issues come to the fore when we think about habitats or ecosystems. From humans’ macroscopic perspective, these are termed aquatic (lentic or lotic) or soil or “extreme” et cetera. But from a microbe’s microscopic perspective, I have argued that there are 4 primary microbial ecosystems (Konopka, 2006) based upon the relevant physical and chemical characteristics at that scale.
Reproduced from Konopka, A. (2006). Microbial ecology – Searching for principles. Microbe , 1 , 175–179.
In relatively still waters such as in freshwater lakes and the ocean, the lifestyle is primarily as free-floating planktonic organisms. Near the surface, there is turbulent mixing driven by wind forces that will impact the light regime experienced by photoautotrophic microbes. Chemoheterotrophs are most often limited by the supply of labile dissolved organic C (DOC). Although the total DOC concentration in ocean waters may approach 1 mg L − 1, this represents the sum of several hundred distinct organic substrates, each present at very low (nmol to μmol L− 1 ) concentrations. The concentrations are low because bacteria have evolved high-affinity transport systems, and competitive forces select for microbes that can exploit multiple substrates at low concentrations.
Other microbes that are found in systems saturated with water must deal with strong concentration gradients of chemical substances, because they are relatively fixed in a solid matrix (such as an aquatic sediment, microscopic biofilm on surfaces in a flowing stream, macroscopic microbial mat or subsurface aquifer). Strong spatial gradients can form in these systems because chemical sources and sinks differ in location. If microbes are sinks or sources for chemical species, their numbers and activity will affect the steepness of the gradients. These gradient systems may be stable in time or show significant temporal fluctuations. For example, microbial mats contain photoautotrophic cyanobacteria in the top few millimeters of the mat and a variety of chemoheterotrophic bacteria are located deeper. Experiments with microelectrodes have shown that there are large and rapid changes in chemical species such as O2 , H2S, and H+ within minutes after sunrise and sunset.
Terrestrial soil habitats that are not saturated with water are akin to vast deserts in which there is an occasional convergence of water, nutrients, and microbial cells on a particle that results in a burst of microbial activity. Soils may seem to be rich with microbes (109 bacterial cells and thousands of different bacterial genomes per gram), but these numbers occupy < 0.01% of the soil volume. The spatial location of microbial activities in soil can be conceptualized as a series of three-dimensional maps that overlie each other within a matrix of pores. These maps describe the spatial location of (1) different microbes (which have distinct biochemical functionalities), (2) chemical resources (organic and inorganic), and (3) water and gases. Each is necessary but none by themselves is sufficient – activity will proceed only where appropriate levels of all necessary factors intersect. The result is a complex three-dimensional landscape in which the physical structure, determined by not only the mineral matrix but also by plant roots, movement of the soil fauna and microbial cells sets the conditions for the activity of microbes and the fate of organic and inorganic C, N, and other elements.
When plants or animals serve as microbial ecosystems, chemical gradients and physical structures such as those enumerated above are important. But an additional very important factor is the capacity for specific molecular interactions between biomolecules of the microbe and those on cells of the plant or animal. A complex interplay via (co)evolution by both the microbe and its “environment” can also be an important factor in these ecosystems.
My intention is to discuss what the field of microbial ecology is and what it should be. I recognize that as a retired research scientist this provides me with certain free-thinking freedoms. I no longer have the fetters of worrying about finishing my dissertation, getting the next research article published, finding a professionally satisfying position or getting that next research grant to support the students and staff in my lab. I hope not to shrink from critical comments on where the field (and I) appear to have fallen short of my ideal.
One of the ways I have filled the gap in my life upon retirement from science is re-immersing myself in photography. In the digital age, there are lots of opportunities for technical approaches to image post-processing. And of course, photography provides opportunity for creative expression. I will try to end each post here with a photograph of mine.